Introduction
When the temperature of a solid or liquid changes, variation in pressure and volume are small and normally neglected. Hence, solids have been assigned a specific heat of one.
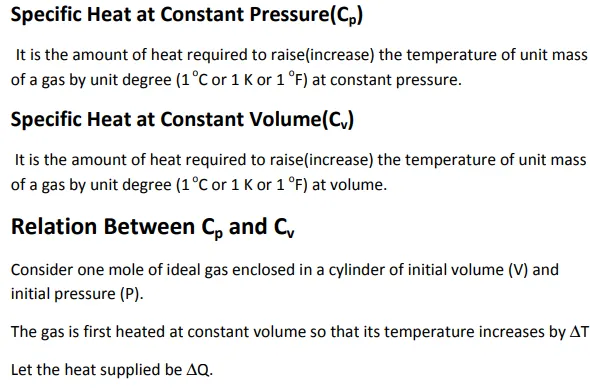
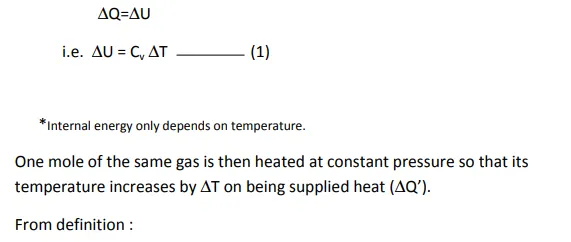
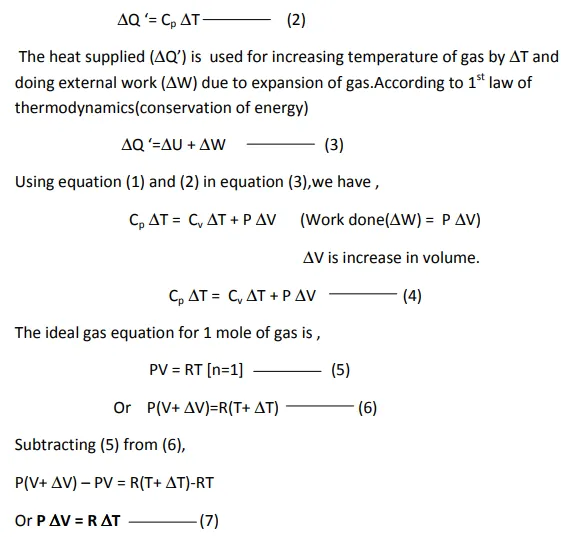
However, when a gas is heated, there is large(significant)change in pressure and volume which cannot be neglected. Hence,each gas has two values of specific heat, one at constant volume(Cv) and another at constant pressure(Cp).





Comments are closed.