The earth contains a large number of metals which are useful to man. One of the most important of these is iron. Modern industry needs considerable quantities of this metal, either in the form of iron or in the form of steel. A certain number of non-ferrous metals, including aluminium and zinc, are also important, but even today the majority of our engineering products are of iron or steel. Moreover, iron possesses magnetic properties, which have made the development of electrical power possible .
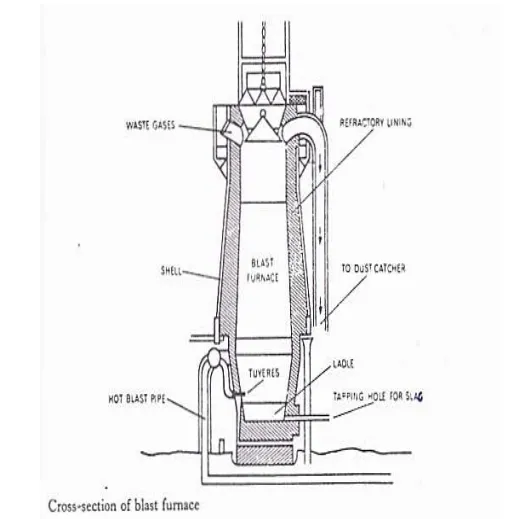
The iron ore which we find in the earth is not pure. It contains some impurities which we must remove by smelting. The process of smelting consists of heating the ore in a blast furnace with coke and limestone, and reducing it to metal. Blasts of hot air enter the furnace from the bottom and provide the oxygen which is necessary for the reduction of the ore. The orebecomes molten, and its oxides combine with carbon from the coke. The non-metallic constituents of the ore combine with the limestone to form a liquid slag. This floats on top of the molten iron, and passes out of the furnace through a tap. The metal which remains is pig-iron.
We can melt this down again in another furnace – a cupola – with more coke and limestone, and tap it out into a ladle or directly into moulds. This is cast-iron. Cast-iron does not have the strength of steel. It is brittle and may fracture under tension. But it possesses certain properties which make it very useful in the manufacture of machinery. In the molten state it isvery fluid, and therefore it is easy to cast it into intricate shapes. Also it is easy to machine it. Cast-iron contains small proportions of other substances. These non-metallic constituents of cast-iron include carbon, silicon and sulphur, and the presence of these substances affectsthe behaviour of the metal. Iron which contains a negligible quantity of carbon, for example wrought-iron, behaves differently from iron which contains a lot of carbon. The carbon in cast-iron is present partly as free graphite and partly as a chemical combination of iron and carbon which we call cementite.This is a very hard substance,and itmakes the iron hard too. However, iron can only hold about 1 % of cementite. Any carbon content above that percentage is present in the form of a flaky graphite.Steel contains no freegraphite, and its carbon content ranges from almost nothing to l %. We make wire and tubing from mild steel with a very low carbon content, and drills and cutting tools from highcarbon steel.






Comments are closed.