Batteries:
When two or more electrochemical cells are electrically interconnected, each of which containing two electrodes and an electrolyte is called a Battery.
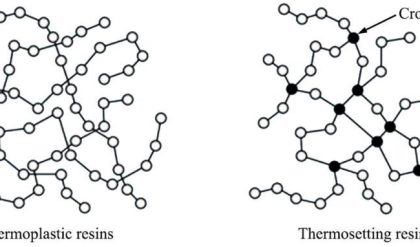
Batteries are classified into a two categories depending on their recharging capabilities.
Primary Batteries: “These are non-rechargeable and are meant for single use and to be discarded after use”.
These are non-reversed and are less expensive and are offer used in ordinary gadgets like torch lights, watches and toys.
Eg: Leclanche cell, Dry cell.
Secondary Batteries: – These are rechargble and are meant for multi cycle use. After every use the electrochemical reaction could be reversed by external application fades or lost due to leakage or internal short circuit. Eg: Lead-acid cell, Ni/cd cell
Differences between Primary and secondary batteries:










Comments are closed.